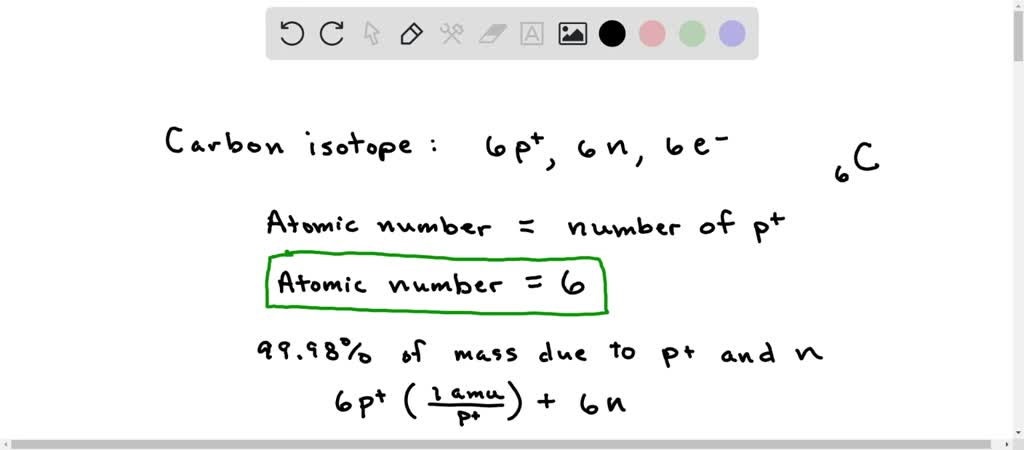
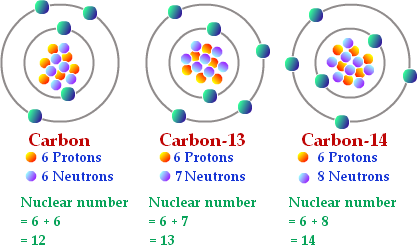
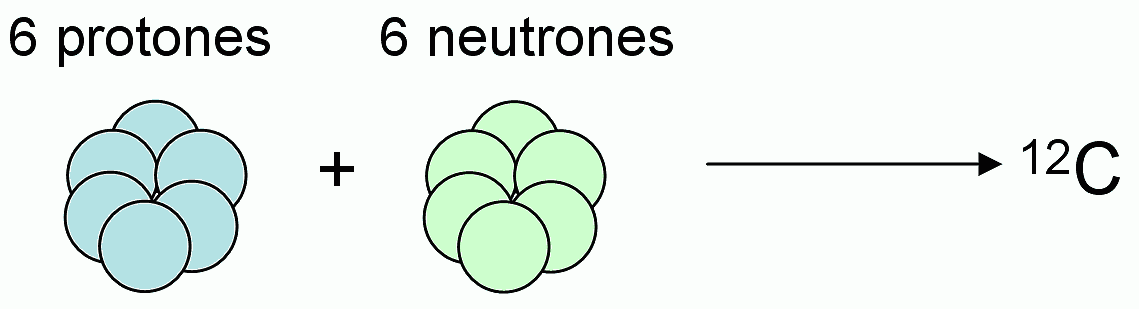
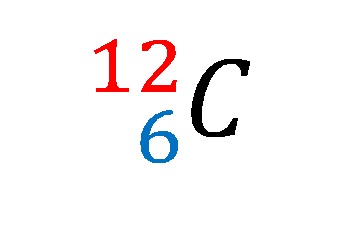Isótopos del carbono: 12 C (6 protones y 6 neutrones), 13 C (6 protones... | Download Scientific Diagram

Isótopos del carbono: 12 C (6 protones y 6 neutrones), 13 C (6 protones... | Download Scientific Diagram

Nuclear Physics The Manhattan Project brings the world into the Nuclear Age. 1 st nuclear test explosion New Mexico, 1945 Test bomb code named “the gadget” - ppt download

How many neutrons and protons are there in the following nuclei? 6^13C, 8^16O, 12^24Mg, 26^56Fe, 38^88Sr

2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines | Circulation

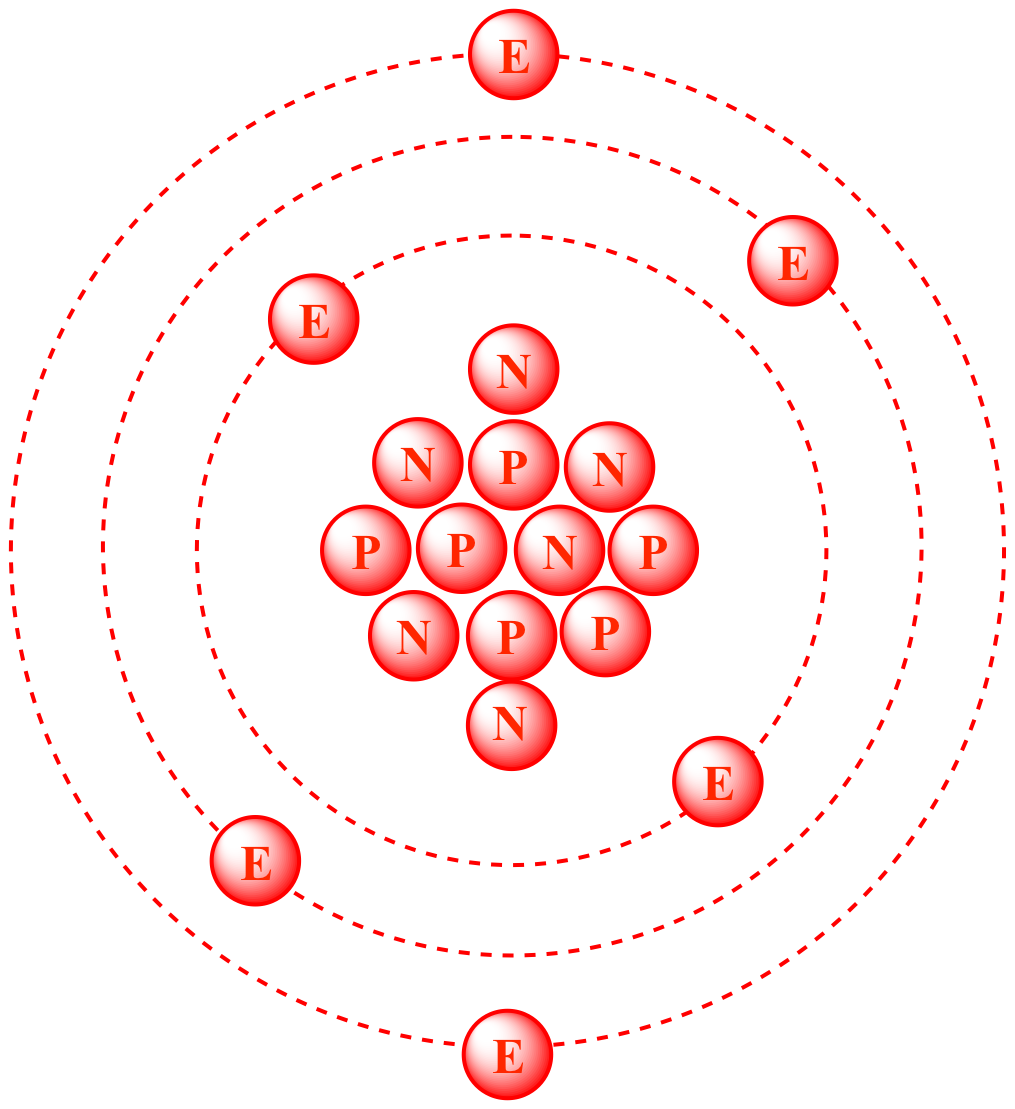
Protons Neutrons Electrons Nucleus n n e e p p p n n Do Now: Draw a model of an atom, label the following: Protons, Neutrons, Electrons, and nucleus. - ppt download

The Atom Date:. Definition of atom: – The basic unit of an element; the building blocks of matter – Have no charge – Composed of 3 subatomic particles. - ppt download

NCERT Exemplar Solutions for Class 6 Science Chapter 12 - Electricity and Circuits free pdf available

1 Elements each element has a unique number of protons in its nucleus the number of protons define the element the number of protons in the nucleus of. - ppt download
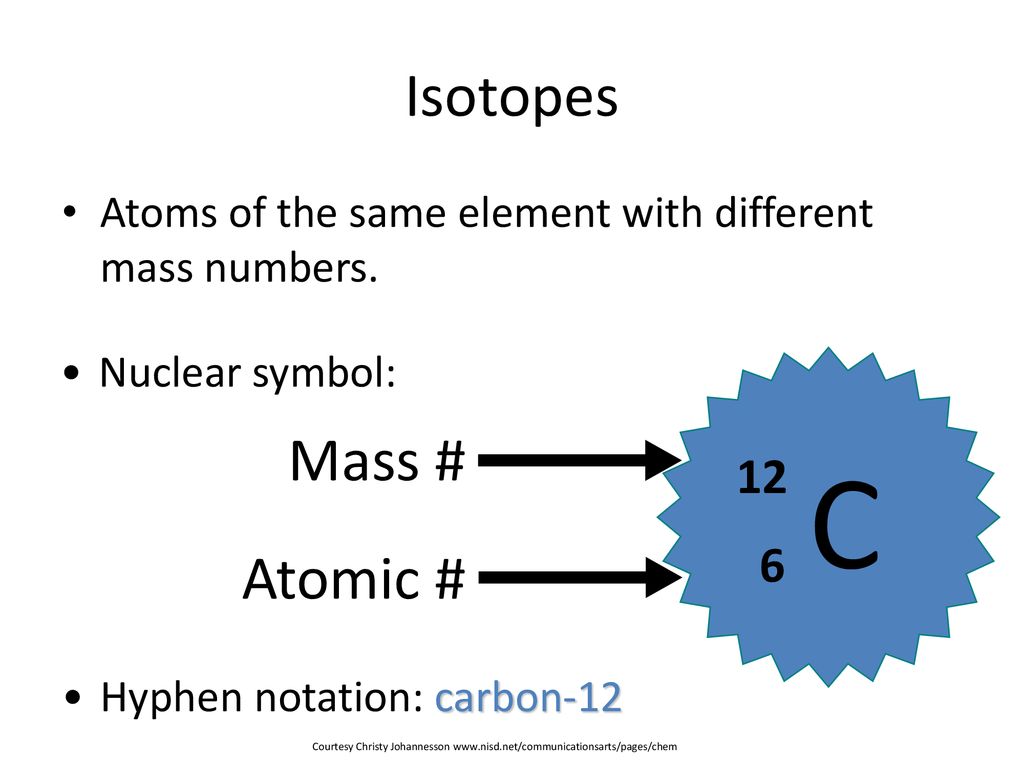
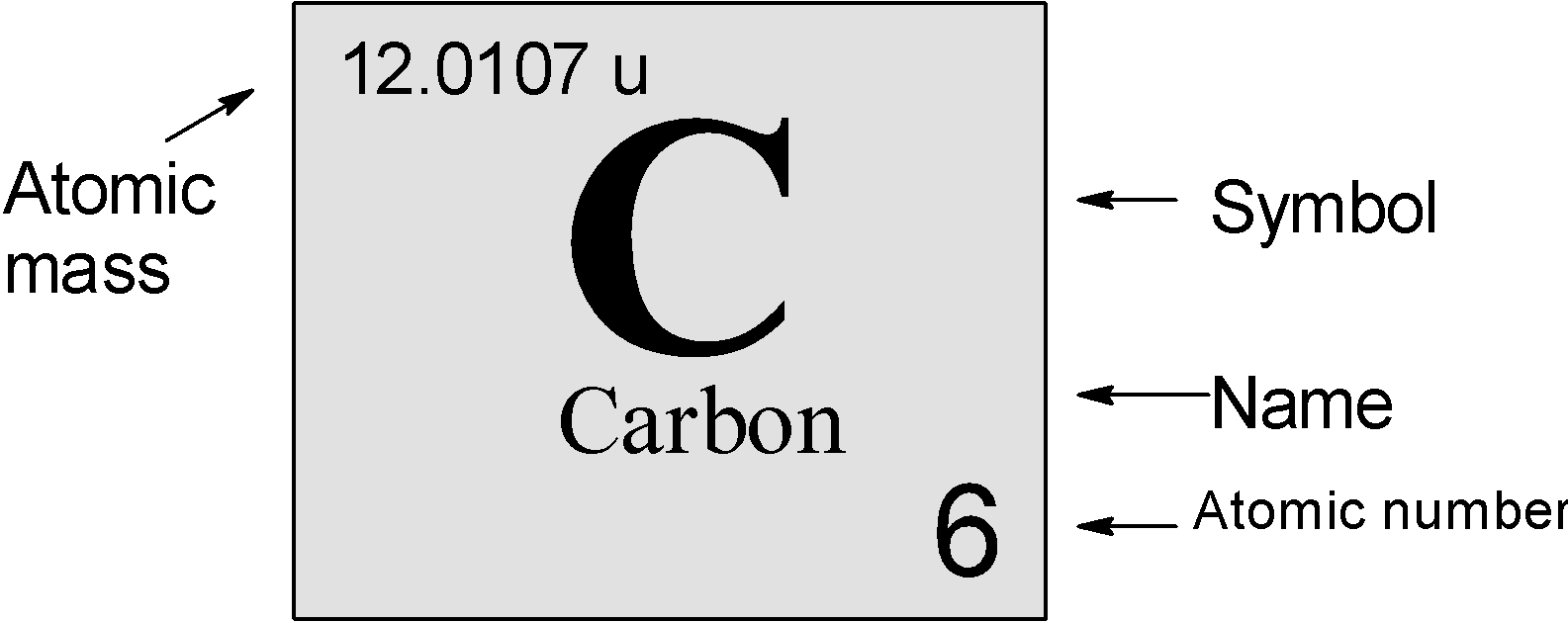
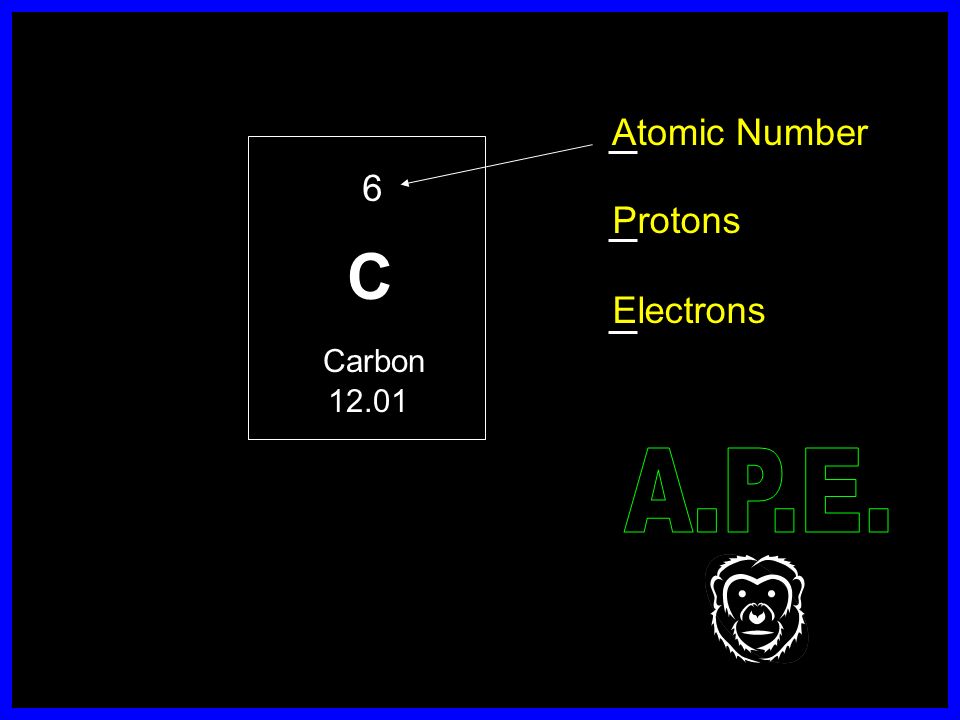
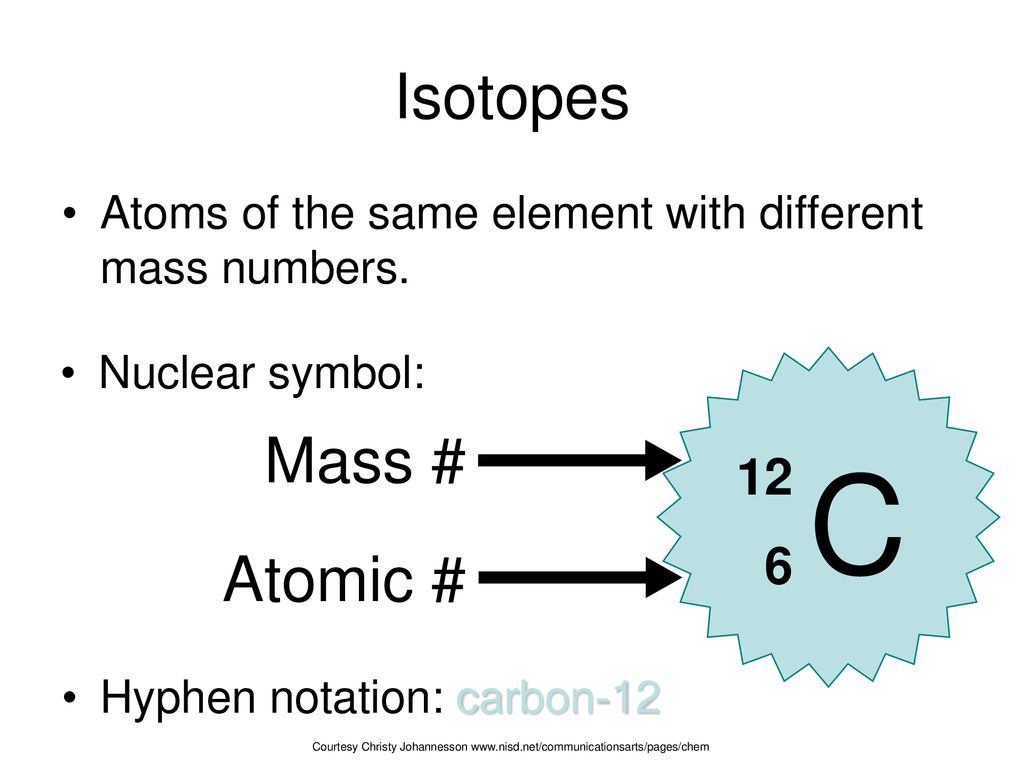
Symbols Contain the symbol of the element, the mass number and the atomic number X Mass number Atomic # protons + # neutrons mass number # protons. - ppt download