EP1421055B1 - Methods for the synthesis of amines such as ephedrine and intermediates - Google Patents

An N-methyltransferase from Ephedra sinica catalyzing the formation of ephedrine and pseudoephedrine enables microbial phenylalkylamine production - Journal of Biological Chemistry

Biotransformation of benzaldehyde to L‐phenylacetylcarbinol (L‐PAC) by Torulaspora delbrueckii and conversion to ephedrine by microwave radiation | Semantic Scholar
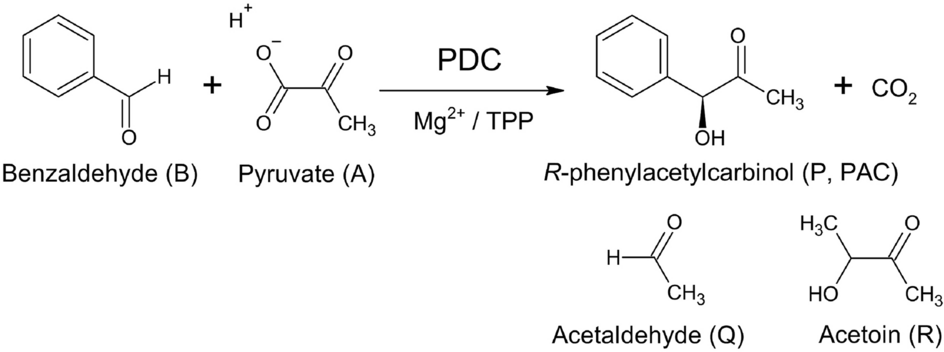
Validation of mathematical model with phosphate activation effect by batch (R)-phenylacetylcarbinol biotransformation process utilizing Candida tropicalis pyruvate decarboxylase in phosphate buffer | Scientific Reports
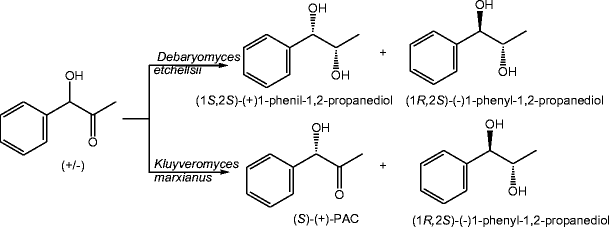
Potential of some yeast strains in the stereoselective synthesis of (R)-(−)- phenylacetylcarbinol and (S)-(+)-phenylacetylcarbinol and their reduced 1,2-dialcohol derivatives | SpringerLink

US7176332B2 - Methods for the synthesis of amines such as ephedrine and intermediates - Google Patents

Asymmetric synthesis of ( S )-phenylacetylcarbinol – closing a gap in C–C bond formation - Green Chemistry (RSC Publishing) DOI:10.1039/C6GC01803C

Potential of some yeast strains in the stereoselective synthesis of (R)-(−)- phenylacetylcarbinol and (S)-(+)-phenylacetylcarbinol and their reduced 1,2-dialcohol derivatives | SpringerLink
Selection of Yeasts for the Production of L-phenyl-acetil- carbinol Bybiotransformation in Shake Flasks

Synthesis of novel β-amino alcohols from phenylacetylcarbinol: cytotoxicity activity against A549 cells and molecular docking | SpringerLink

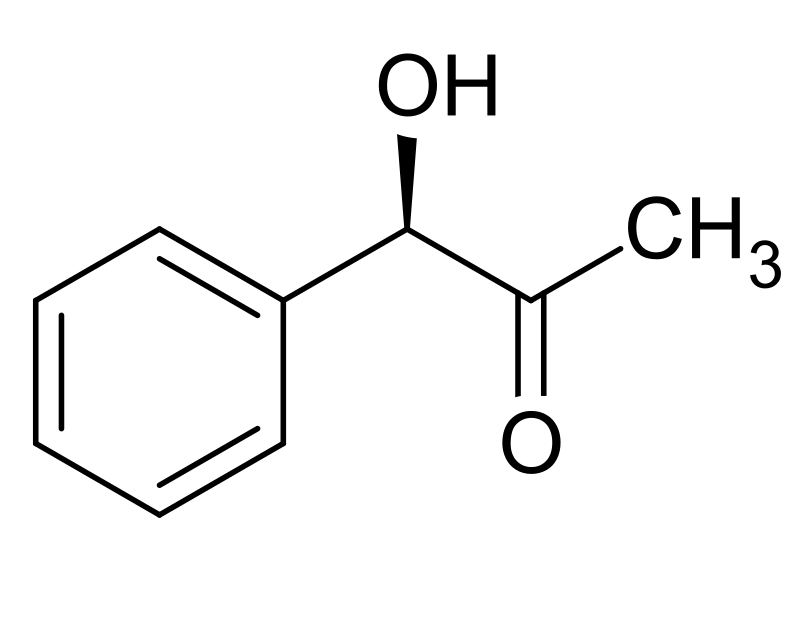
Synthesis of R-(−)-phenylacetylcarbinol by fermenting yeast and... | Download High-Resolution Scientific Diagram
Raney-nickel-iron catalyst, its preparation and a method to produce L-norephedrine by hydrogenating L-phenylacetylcarbinol-oxime with said catalyst - Patent 2055379

Improvement of the yeast based (R)-phenylacetylcarbinol production process via reduction of by-product formation - ScienceDirect

DE60226038T2 - PROCESS FOR SYNTHESIS OF AMINES SUCH AS EPHEDRINE AND INTERMEDIATE PRODUCTS - Google Patents
Sciencemadness Discussion Board - Synthesis of Phenylacetylcarbinol by Alkyne Hydration and Subsequent Enamine formation - Powered by XMB 1.9.11
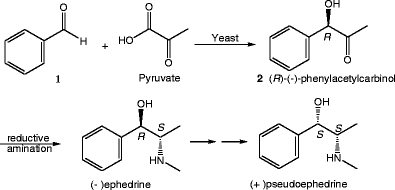
Potential of some yeast strains in the stereoselective synthesis of (R)-(−)- phenylacetylcarbinol and (S)-(+)-phenylacetylcarbinol and their reduced 1,2-dialcohol derivatives | SpringerLink

Investigation of the l-phenylacetylcarbinol process to substituted benzaldehydes of interest - ScienceDirect

Catalytic Cycle of Rhodium-Catalyzed Asymmetric 1,4-Addition of Organoboronic Acids. Arylrhodium, Oxa-π-allylrhodium, and Hydroxorhodium Intermediates | Journal of the American Chemical Society

Mechanistic study of the biosynthesis of R-phenylacetylcarbinol by acetohydroxyacid synthase enzyme using hybrid quantum mechanics/molecular mechanics simulations - ScienceDirect

US7176332B2 - Methods for the synthesis of amines such as ephedrine and intermediates - Google Patents